Summary
- While GALT failed the phase 2 NASH-CX trial, they demonstrated a clear benefit for patients without esophageal varices.
- To date, belapectin is the only candidate to demonstrate a positive benefit in NASH cirrhosis patients.
- The $20M investment by the Chairman also supports the long thesis.
On Tuesday, December 10, Galectin Therapeutics (NASDAQ:GALT) closed at $2.79/share, very near its 52-week low of $2.74/share. The recent drift down from $4.00+/share began on November 12, when they reported 3Q19 results. While the financials contained nothing material, it was revealed that the design of the highly-anticipated phase 3 trial of lead candidate belapectin in NASH cirrhosis was being refined due to ongoing input from the FDA. Management said that the FDA seemed to be departing from its earlier implied potential support of a certain surrogate endpoint for accelerated approval. Communication between the two is still ongoing, but investors did not like the added delay and punished the stock accordingly.
While we think that the long gap between the completion of phase 2 and the start of phase 3 could have been shortened with better execution, we are still very bullish on the long-term prospects of GALT. The aim of this article is simply to remind investors of the value in GALT’s pipeline.
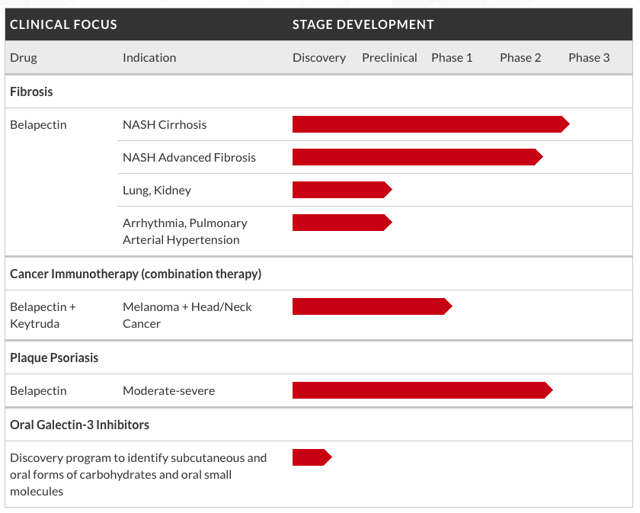
GALT’s only candidate past the preclinical stage is belapectin, a new chemical entity that targets galectins such as galectin-1 and galectin-3. A quick glance at their pipeline shows us that belapectin is in several potentially promising trials.
GALT: Development Pipeline
Source: Company website
It’s in a phase 2 trial for moderate-to-severe plaque psoriasis, as well as a phase 1 combo trial with Merck & Co.’s (NYSE:MRK) Keytruda for melanoma and head/neck cancer. But the real prize is the NASH indication.
Nonalcoholic steatohepatitis, or NASH, is an illness that involves fat build-up in the liver that can lead to severe scarring called fibrosis. Left untreated, patients can eventually develop cirrhosis, which involves the permanent loss of liver cells, and may require a liver transplant. Current estimates put the NASH population in the US at upwards of 15M, with that number expected to explode over the next 10 years, driven by the rise in diabetes and obesity. To date, there are no approved therapies for NASH. But given the expected $20B-$35B market, the field of companies conducting clinical trials is very crowded.
One of the reasons we like GALT so much is because they have managed to show positive results in a clinical trial involving NASH cirrhosis patients that have yet to develop esophageal varices. Esophageal varices are abnormally enlarged veins in the esophagus that can cause serious complications from bleeding. They can develop when normal blood flow to the liver is disrupted by liver scarring.
Liver damage can occur very slowly, meaning that patients can live with the condition for several years without noticing any symptoms. But once they reach the later-stages of cirrhosis, it becomes very difficult to treat.